
Available Methods and Accessories
The AFM user facility residing within IMS excels at multiple advanced characterization methods. Lab personnel have substantial expertise with a wide range of novice to expert AFM techniques that utilize creative methods to achieve imaging needs.
Accessories include liquid cell with RT up to 40C temperature control, POLY heater for variable temperature AFM (RT up to 300C), humidity control via salt solutions, and all hardware and software for nanolithography, contact and AC mode (‘Tapping’) imaging, and contrast mechanisms spanning phase, CAFM, pcAFM, EFM, MFM, SSPM/KPFM, PFM, AFAM, FFM, and F-d measurements and mapping.
For independent or even simultaneous epi-fluorescence, the system is mounted on a Nikon TE2000 inverted optical microscope featuring z-stacking. It includes: Hamamatsu Flash4v3 camera; PI focal plane controller (100µm max range with nm accuracy); automated microshutters; several objectives (10x, 40x, 60x oil, 100x); extra 1.5x magnifier available; illumination by either i) a phosphor based broad-band visible LED, or ii) a Hg arc lamp (Lambda DG4) with automated filter control among 4 selections; filter wheel with 5 selectable dichroics including a dual window (R/G) filter, and software (Volocity by Improvision / Perkin Elmer) for 3D optical imaging, deconvolution, and feature tracking.
Sample Requirements
- Conventional AFM: Optimal samples up to 1cm by 1cm (xy) and 5mm (z). Up to 5cm by 5cm (xy) and 2cm (z) can be accommodated under certain conditions. Samples will be fixed to center of a standard glass microscope slide (25mm by 75mm).
- Epi-fluorescence imaging: Samples must adhere to center of either a thin glass or Thermanox coverslip (25 or 35 mm diameter, #1.5 thickness), OR a thin glass-bottomed dish (35mm diameter total, 14 mm glass diameter, #1.5 thickness).
Summary of Technique
- Conventional AFM:
- AFM can provide high resolution imaging of the surface topography, and for certain specimens also local materials properties. These can include phase, current, photocurrent, electric fields, magnetic fields, surface potential, piezoresponse, mechanical compliance, friction, adhesion, and stiffness. Tomographic AFM may even be feasible for certain applications.
- Note AFM is generally slow and difficult, requiring many hours for quality data and tens of hours of practice to begin to master.
- Epi-fluorescence imaging: Filterable light source illuminates sample from below, then detects fluorescence also from below.
- It is feasible, albeit very difficult, to achieve both AFM and fluorescence simultaneously.
Information Provided & Detection Limits
- Conventional AFM: Max AFM scan range = 100µm by 100µm (xy) by 15µm (z). Typical realistic resolution (accommodating tip contact area and depending on contrast mechanism) is 1to 50 nm (xy) and a few Angstroms in (z). For oscillating signals, tens of pm resolution is practical. Common results: Images, point measurements, roughness calculations, height or property histograms. Original data format: Wavemetrics Igor (.ibw).
- Epi-fluorescence imaging:
- The table below summarizes individual pixel sizes and overall fields of view for common microscope configurations, based on our camera specs of 2048x2048 pixels, 6.5µm by 6.5µm each, 37000:1 dynamic range, 0.006 e-/pixel/s (Water Cooled to -30° C). Max z-ranges are typically limited by objective working distance, scattering, and/or autofluorescence. Common results: Images, image Stacks, intensity histograms, feature tracks. Original data format: Images and stacks (.tif); feature tracking (.xlsx).
- Objective and Camera Details:
- The table below summarizes individual pixel sizes and overall fields of view for common microscope configurations, based on our camera specs of 2048x2048 pixels, 6.5µm by 6.5µm each, 37000:1 dynamic range, 0.006 e-/pixel/s (Water Cooled to -30° C). Max z-ranges are typically limited by objective working distance, scattering, and/or autofluorescence. Common results: Images, image Stacks, intensity histograms, feature tracks. Original data format: Images and stacks (.tif); feature tracking (.xlsx).
| Objective: | 10x | 40x | 60x (oil) | 100x |
| nm/pix | 650 | 163 | 108 | 65 |
| with 1.5x mag | 433 | 108 | 72 | 43 |
| xy range (um) | 1331 | 333 | 222 | 133 |
| with 1.5x mag | 887 | 222 | 148 | 89 |
| Hamamatsu Flash4v3: | pix/side: | 2048 | um/pix: | 6.5 |
-
- The tables below summarize excitation and emission wavelengths for which the system is typically configured. Other options can possibly be accommodated for users who can supply the appropriate filters.
- Microscope Filter Wheel:
- The tables below summarize excitation and emission wavelengths for which the system is typically configured. Other options can possibly be accommodated for users who can supply the appropriate filters.
| filter wheel label | Excitation Bandwidth (nm) | Emission Bandwidth (nm) | Part # (real, or cloesest available in 2024) |
| UV | 340-380 (UV) | 435-485 (indigo+blue) | Nikon 96310 UV-2E/C C63936 |
| B | 465-495 (blue) | 515-555 (green) | Nikon B-2E/C, 96311, C65357 |
| G | 528-553 (green) | 590-650 (orange) | Nikon G-2E/C, 96312, C65358 |
| arrows (actually dual: B/G) | CFP, or GFP/YFP: 432-440, 488-510 | 453-480, 525-566 (blue/green) | Chroma 52017 CFP/YFP |
| Bright Field | full spectrum | >420 (UV filtered) | Chroma: 33002, BFT FLD, C53823 |
| G/R | Dual: 458-499, 570-600 (blue/yellow) | 507-541, 610-648 (green/red) | Semrock FITC/Txed-2x-a-nte. EXCITE: FF01-479/585-25, EMIT: FF01-524/628-25 |
-
-
- DG-4 (Hg Arc Lamp and 4 automated filter sets):
-
| Improvision label | TxRed | GFP | Open | CFP |
| low edge (nm) | 576 | 475 | UV | 430 |
| high edge (nm) | 596 | 495 | near IR | 440 |
| filtered color | yellow | blue | full spectrum (Hg arc lamp) | purple |
| Note1: YFP (490-510 nm excitation) may be swappable into 1 of the 4 slots on request. | ||||
| Note2: a manual mirror can be flipped for white light (phosphor LED) excitation instead. | ||||
- Deconvolution:
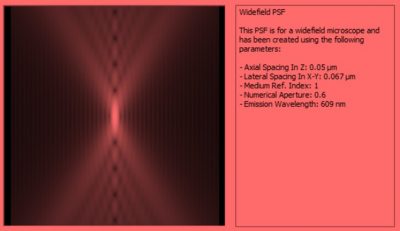
- We use Perkin Elmer’s Improvision Volocity software package to perform deconvolution with z-stacks when applicable. An example of the widefield psf for our 40x air objective at 609nm is shown below.
Lab Location and Contact Information
Lab Location: AFM user facility, Science1, G024
Lab Manager: Professor Bryan Huey
bryan.huey@uconn.edu
860-486-3284